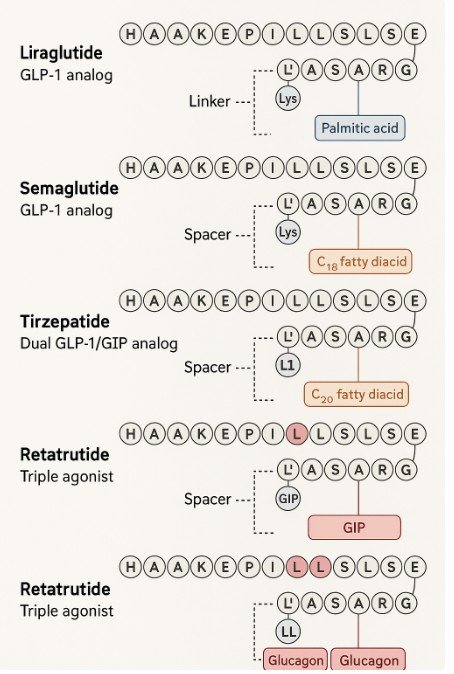
Long-Term Effects of GLP-1 Receptor Agonists
Benefits (well supported by evidence)
- Weight reduction: Sustained weight loss of 10–20% is common with higher-dose semaglutide and tirzepatide (dual GIP/GLP-1). This appears durable with ongoing treatment.
- Cardiovascular protection: Several GLP-1 RAs (liraglutide, semaglutide, dulaglutide) have shown reduced risk of major adverse cardiovascular events (MACE: CV death, nonfatal MI, nonfatal stroke) in high-risk patients.
- Kidney outcomes: Trials (e.g., FLOW for semaglutide) suggest slowed progression of diabetic kidney disease, with reductions in albuminuria and preservation of eGFR.
- Metabolic control: Durable lowering of HbA1c with low hypoglycemia risk (since glucose-dependent mechanism).
- Fatty liver disease (NASH/NAFLD): GLP-1s improve liver enzymes, reduce hepatic fat, and early evidence suggests histological improvement in NASH.
Risks / Considerations (long-term safety)
- GI side effects: Nausea, vomiting, constipation are the most common. Usually improve over time but may persist in some patients.
- Gallbladder disease: Slightly increased risk of gallstones and cholecystitis, possibly due to rapid weight loss and altered bile metabolism.
- Pancreatitis: Historically a concern; large meta-analyses suggest only a small, if any, increased risk. Still monitored closely.
- Thyroid tumors: In rodents, GLP-1 RAs caused medullary thyroid carcinoma. Human data don’t show a clear risk, but contraindicated in patients with MEN2 or medullary thyroid carcinoma history.
- Muscle and bone health: Rapid weight loss can cause loss of lean mass; long-term monitoring of sarcopenia and bone density is being studied.
- “Maintenance effect”: Weight regain is common if the drug is stopped, highlighting that long-term (possibly lifelong) therapy may be necessary for sustained benefit.
Emerging Evidence: Ozempic (Semaglutide) & Eye Complications
The main area of focus has been diabetic retinopathy (DR) progression.
What has been observed
- In the SUSTAIN-6 trial (semaglutide in type 2 diabetes, 2016), there was an increased rate of diabetic retinopathy complications (vitreous hemorrhage, blindness, need for photocoagulation or intravitreal agents) in the semaglutide group compared to placebo (3.0% vs. 1.8%).
- Importantly, this was most pronounced in patients with pre-existing retinopathy and very rapid, large HbA1c reductions (e.g., >1.5–2% within months).
Hypothesis for mechanism
- “Early worsening phenomenon”: Similar to what has been seen historically with insulin initiation. When glucose improves too quickly in patients with long-standing poor control, retinal microvasculature may destabilize and worsen before ultimately improving.
- Not believed to be a direct toxic effect of semaglutide on the retina.
Recent data & nuance
- Longer-term follow-up and real-world data suggest the retinopathy risk may level off after the first year.
- A 2022 meta-analysis did not find a consistent increased risk of new or worsening retinopathy across all GLP-1 RA trials, suggesting the effect may be specific to rapid HbA1c drops in high-risk patients.
- Ongoing trials (like FOCUS, assessing semaglutide and diabetic retinopathy progression) are expected to clarify whether semaglutide independently worsens DR or if the risk is transient.
Clinical takeaways
- Patients with advanced diabetic retinopathy should be monitored closely when starting Ozempic/Wegovy or other GLP-1s, especially if rapid glucose lowering is expected.
- A slower dose titration and staged approach to glycemic control may help mitigate the risk.
- For patients without pre-existing DR, the risk appears low.
✅ Summary:
Long-term, GLP-1 receptor agonists show strong evidence for weight loss, CV protection, kidney benefit, and possible liver improvement. Main risks are GI issues, gallbladder disease, possible lean mass loss, and rare concerns with pancreas/thyroid. Regarding Ozempic and the eyes, the signal of worsening diabetic retinopathy seems linked to rapid HbA1c reduction in patients with existing retinopathy, rather than a direct drug toxicity. Monitoring and gradual glycemic improvement remain key until ongoing studies provide more definitive guidance.
There is also some emerging anecdotal evidence on more serious eye issues with Ozempic, research is ongoing, this complication is called non-arteritic anterior ischemic optic neuropathy (NAION).