Ketamir-2: A Promising Oral Ketamine Analog Poised to Shift Pain and Mood Treatment
Introduction
In recent years, ketamine has emerged from its anesthesia roots to become a promising treatment for conditions like treatment-resistant depression, suicidal ideation, and neuropathic pain. But it comes with trade-offs: side effects, poor oral absorption, and concerns about safety. A few that I’ve even seen in my clinical practice after a patient got a large infusion at an outpatient clinic, something called “ketamine bladder” – an interesting read for another day.
Enter Ketamir-2 (also just “Ketamir”), a new molecular entity designed to keep the benefits while reducing many of the drawbacks. It’s still early days in the research (preclinical), but the data are intriguing.
So how did i stumble across this medication so early in development? A patient had asked me to look into medications for neuro related pain, he has been (and still is) suffering with post herpetic neuralgia for nearly two decades and has tried EVERY medication in the book. So down the rabbit hole i went.
What Is Ketamir-2?
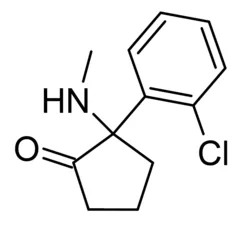
Ketamir-2 is an analog of ketamine—an NMDA receptor antagonist—but with some structural tweaks. Instead of ketamine’s six-membered cyclohexane ring, Ketamir-2 employs a five-membered cyclopentane ring. This might sound technical, but the shift leads to better selectivity, lower affinity to the receptor, improved oral bioavailability, and a better safety profile. Frontiers
It binds selectively to the PCP (phencyclidine) site on the NMDA receptor, with an IC₅₀ of about 100 µM. For comparison, ketamine has much higher (i.e. tighter) binding affinity, with IC₅₀/Ki in the submicromolar range.
Ketamir-2’s major metabolite, called desmethylketamir (or “nor-Ketamir”), also targets the PCP site, but with even lower affinity (≈ 470 µM). Frontiers
Importantly, in vitro screening across dozens of receptors, transporters, enzymes, and ion channels has found no significant off-target interactions for Ketamir-2 until relatively high concentrations—where ketamine would usually hit many more sites. That suggests a cleaner pharmacological profile. Frontiers
Why Researchers Are Paying Attention: Preclinical Evidence
Oral Neuropathic Pain Models
Pain, especially neuropathic pain, is notoriously difficult to manage. Ketamir-2 has shown in animal studies (rats and mice) that oral administration, particularly in its pamoate salt formulation, reduces pain in well-established models:
- Spinal nerve ligation (Chung’s model) in rats: Ketamir-2 substantially increased withdrawal thresholds (i.e., reduced pain sensitivity) at moderate to high doses. Ketamine in parallel did not perform as well at similar doses. Frontiers
- Paclitaxel-induced neuropathy model in mice: Ketamir-2 again showed strong effects, outperforming or matching positive control drugs like gabapentin and pregabalin in many measures. Frontiers
Safety and Side Effect Profile
One of the biggest criticisms of ketamine is its side effects—dissociation, sedation, psychotomimetic effects, potential for abuse. Ketamir-2 seems to sidestep many of those, at least in animal work:
- It does not induce hyperlocomotion (the animal-behavior sign often tied to psychomotor agitation) when given orally. Ketamine usually does. Frontiers
- Neuropathic pain models show pain relief without overt sedation or disorientation in animals. Frontiers
- Pharmacokinetics (PK) studies suggest rapid absorption, a short half-life, and good brain penetration, with lower systemic binding. Frontiers
Where Things Stand Now: Regulatory & Clinical Path
Although most data are preclinical, some regulatory steps are underway:
- The FDA has cleared an Investigational New Drug (IND) application for Ketamir-2 in neuropathic pain, based on preclinical safety, pharmacology, and toxicology. Psychiatric Times
- There are multiple animal studies demonstrating efficacy, and plans for human trials are advancing (e.g. in diabetic peripheral neuropathy, chemotherapy-induced neuropathy). Psychiatric Times
- Also noteworthy: Ketamir-2 is considered not a controlled substance under current U.S. regulations, which may simplify regulatory and market pathways compared to ketamine. Wikipedia
Possible Advantages, Unanswered Questions
What Looks Good
- Oral administration: If human trials replicate animal results, oral dosing would be a big win (more convenient, wider potential use) compared to IV ketamine.
- Cleaner side effect profile: Less risk of sedation, dissociation, abuse potential—if animal behavior translates.
- Selectivity: Hitting the PCP site specifically, avoiding many other receptor systems, may make for fewer off-target adverse effects.
- Multiple formulations: Oracle work suggests oral and topical forms are being developed. A topical version could deliver pain relief locally, with minimal systemic exposure. BioSpace
What We Don’t Yet Know
- Human safety in targeted populations: Animal toxicology is supportive, but effects in people (especially long-term) are unknown.
- Efficacy in depression, anxiety, or psychiatric settings: Preclinical mood/anxiety-like findings are interesting; whether Ketamir-2 can replicate ketamine’s rapid antidepressant effects needs clinical trials. Frontiers
- Dose optimization and side effect thresholds in humans: Where is the sweet spot between efficacy and tolerability? Differences in metabolism across species complicate predictions.
Implications & Outlook
If Ketamir-2 lives up to its promise, it could represent a meaningful advance in pain management (especially neuropathic pain) and possibly mood disorders. For patients suffering from chronic neuropathy, conditions resistant to standard treatments, or for whom existing options have intolerable side effects, this could widen the therapeutic toolbox.
However, caution is wise: many compounds show promise in rodents but don’t always have the same impact in people. Clinical trials will be the real proof. Until then, physicians, patients, and investors will be watching closely.
Conclusion
Ketamir-2 is emerging as a strong contender in the next generation of ketamine analogs—one designed to preserve ketamine’s therapeutic effects while reducing its liabilities. Early preclinical evidence supports its analgesic potency, improved safety, selective binding profile, and oral (and topical) utility. But as with all drug development, the key will be carefully designed clinical trials, dose finding, safety monitoring, and real-world outcomes.
Fingers crossed, maybe 5-10 years in the future we will have a robust medication to treat our patients in pain.
A link to the original article Ketamir