VK2735: How It Differs from Other Obesity and Diabetes Medications
The last few years have seen a surge of powerful new treatments for obesity and type 2 diabetes, with names like semaglutide (Ozempic®, Wegovy®) and tirzepatide (Mounjaro®, Zepbound®) becoming household terms. These drugs, which act on incretin pathways like GLP-1 and GIP, have revolutionized the way we approach weight management and blood sugar control. Another promising addition to this lineup is VK2735.
Now, another contender is making headlines: VK2735, a novel therapy from Viking Therapeutics. Still in clinical trials, VK2735 has shown striking early results in weight reduction—and it may even offer advantages over its competitors. But what exactly is VK2735, and how does it differ from existing medications? Let’s break it down.
What is VK2735?
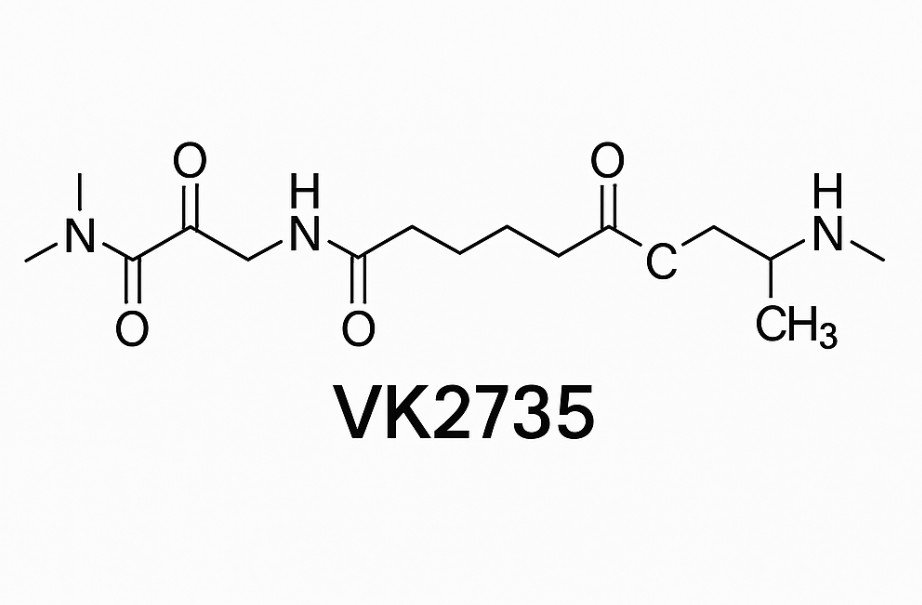
VK2735 is a dual GLP-1/GIP receptor agonist, belonging to the same therapeutic class as tirzepatide. It is designed to:
- Suppress appetite and slow gastric emptying (via GLP-1 activation)
- Improve insulin sensitivity and enhance fat metabolism (via GIP activation)
In other words, it combines the best of both incretin hormones into one therapy.
But Viking Therapeutics isn’t stopping there—VK2735 is being developed in two formulations:
- Injectable VK2735 – a once-weekly subcutaneous injection, similar to semaglutide and tirzepatide.
- Oral VK2735 – a pill formulation currently being tested, which could offer unmatched convenience compared to existing drugs.
VK2735 vs. Other Medications
1. VK2735 vs. Semaglutide (Ozempic, Wegovy, Rybelsus)
- Semaglutide: A GLP-1 receptor agonist only. It reduces appetite, slows digestion, and improves blood sugar, but does not engage GIP pathways.
- VK2735: A dual GLP-1/GIP agonist, potentially offering superior weight loss and improved metabolic benefits compared to semaglutide.
👉 Key Difference: VK2735 uses dual action instead of single receptor targeting. Early data suggests this may translate to greater fat reduction and metabolic improvements.
2. VK2735 vs. Tirzepatide (Mounjaro, Zepbound)
- Tirzepatide: Currently the gold standard, acting as a GLP-1/GIP dual agonist with record-setting weight loss results in clinical studies.
- VK2735: Works in the same dual receptor class, but Viking is testing an oral version, something tirzepatide currently lacks.
👉 Key Difference: While both drugs share a mechanism, VK2735 could become the first oral GLP-1/GIP dual agonist, eliminating the injection barrier for patients.
3. VK2735 vs. Retatrutide
- Retatrutide: A triple agonist (GLP-1, GIP, and glucagon receptors), currently showing the most dramatic weight loss results of all.
- VK2735: A dual agonist (GLP-1 + GIP).
👉 Key Difference: Retatrutide adds glucagon activation, which boosts energy expenditure and fat burning, while VK2735 focuses on appetite suppression and insulin sensitivity. VK2735 may end up being better tolerated with fewer side effects.
4. VK2735 vs. Pemvidutide
- Pemvidutide: A GLP-1 + glucagon dual agonist, targeting appetite suppression and fat burning.
- VK2735: A GLP-1 + GIP dual agonist, targeting appetite suppression and insulin sensitivity.
👉 Key Difference: Pemvidutide emphasizes energy expenditure and liver fat reduction, while VK2735 emphasizes insulin signaling and glucose control. Both may serve slightly different patient populations.
5. VK2735 vs. Orforglipron
- Orforglipron: A non-peptide, oral-only GLP-1 agonist. Designed to survive digestion and offer a daily pill alternative.
- VK2735: Peptide-based, but being developed in both injectable and oral forms.
👉 Key Difference: VK2735 activates two incretin receptors (GLP-1 + GIP), while orforglipron focuses on GLP-1 only.
Why VK2735 is Generating Buzz
- Impressive Early Weight Loss Results
- Phase 1 trials showed patients losing up to 7–8% of body weight in just 28 days, which is remarkably fast compared to other drugs at early stages.
- Longer studies will clarify sustainability, but initial outcomes hint at potency.
- Potential Oral Option
- If successful, an oral dual agonist could make VK2735 a game-changer for patients hesitant about injections.
- Dual Hormone Benefits
- GLP-1 = appetite suppression + blood sugar control
- GIP = improved fat metabolism + insulin sensitivity
- Together, they could provide synergistic weight loss and metabolic health improvements.
- Tolerability and Side Effects
- Like other incretin drugs, the most common side effects are nausea, vomiting, diarrhea, and constipation.
- Some reports suggest VK2735 may cause less severe GI issues than semaglutide or tirzepatide, but more trials are needed.
VK2735 and the Future of Obesity Care
VK2735 could carve out a unique role in the incretin drug market:
- If the injectable form proves equal or superior to tirzepatide, it may compete head-to-head as another dual agonist option.
- If the oral version succeeds, VK2735 could outshine competitors by offering the first easy-to-take pill in the dual agonist class.
This dual strategy positions Viking Therapeutics to potentially serve two types of patients: those comfortable with injections and those who strongly prefer pills.
Who Might Benefit Most from VK2735?
- Obesity patients seeking rapid and significant weight reduction.
- Type 2 diabetes patients needing improved blood sugar control and insulin sensitivity.
- Patients hesitant about injections, who could benefit from the oral formulation.
- People with metabolic syndrome, high cholesterol, or insulin resistance.
The Bottom Line
VK2735 is still in the trial phase, but its dual GLP-1/GIP activity and potential oral delivery set it apart from current medications. While semaglutide and tirzepatide have proven track records, VK2735 may represent the next evolution in incretin therapy, offering powerful results in a more patient-friendly format.
If Phase 2 and Phase 3 results confirm early findings, VK2735 could soon join the ranks of blockbuster obesity medications—and might even become the first oral dual agonist therapy on the market.
Key Takeaways
- VK2735 is a novel dual GLP-1/GIP receptor agonist showing promising early results in weight reduction and potentially better metabolic benefits than existing medications.
- It is available in both injectable and oral formulations, making it versatile for different patient preferences.
- VK2735 may outshine competitors like semaglutide by offering superior weight loss and insulin sensitivity improvements due to its dual action.
- Initial trials show rapid weight loss of 7-8% in just 28 days, hinting at its efficacy and potential as a new player in obesity care.
- If successful, VK2735 could become the first oral dual agonist therapy, addressing the needs of patients hesitant about injections.
Estimated reading time: 5 minutes