What Is Retatrutide?
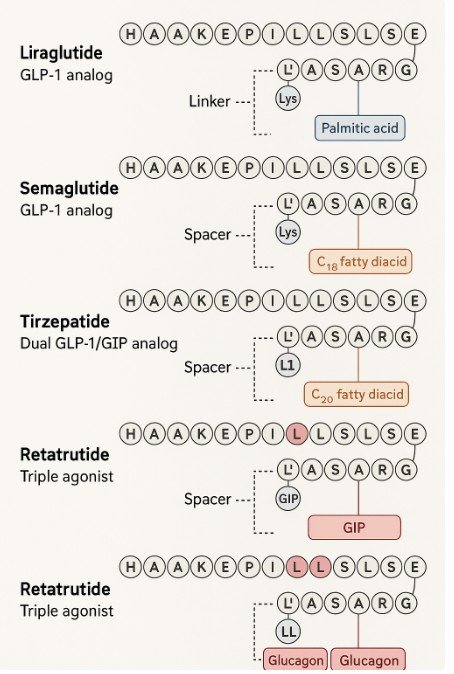
Retatrutide (LY‑3437943) is an investigational weight‑loss agent developed by Eli Lilly. It’s a triple‑agonist, simultaneously targeting the GLP‑1, GIP, and glucagon receptors—unlike existing therapies that target only one or two pathways The Sun+15Wikipedia+15Business Insider+15.
Current Research & Clinical Data
Phase 2 Trial Success
- Published in The New England Journal of Medicine (June 2023), phase 2 trial results revealed impressive outcomes: up to 17.5% mean weight loss at 24 weeks, and 24.2% at 48 weeks, with the highest dosage the biostation+5Drugs.com+5Wikipedia+5.
Additional Health Benefits
- Participants also showed improvements in blood pressure, blood sugar, and liver fat reduction—suggesting benefits beyond weight loss, especially for those with fatty liver disease (MASLD) Verywell Health+1.
Phase 3 Trials Underway
- A Phase 3 clinical trial (for severe obesity and cardiovascular disease) began May 30, 2023, spans around 113 weeks, and continues as of mid‑2025 TipRanks.
- Reports confirm the drug is still in Phase 3 trials and not yet approved PMC+11Drugs.com+11New York Post+11.
Possible Release Timeline
- Business Insider suggests the earliest possible market entry could be late 2026, with 2027 being more likely Business Insider.
- A broader review anticipates launch by or before 2030, positioning retatrutide among top obesity drugs of the decade The Sun+15DelveInsight+15the biostation+15.
- Another estimate puts FDA approval likely after Phase 3 concludes in 2026 Drugs.com+1.
Summary Timeline:
- Phase 2 data: mid‑2023
- Phase 3 ongoing: 2023–2025+
- FDA approval: potentially 2026–2027, depending on trial results and regulatory review
Potential Impact on the GLP-1 Market
Unparalleled Efficacy
- Retatrutide’s ~24% average weight reduction in under a year outpaces semaglutide (Wegovy/Ozempic) and tirzepatide (Mounjaro/Zepbound), which achieve ~15% and ~21% losses, respectively Lilly Trials+3Heally+3Wikipedia+3Business Insider+2New York Post+2PMC+14The Times+14Heally+14.
- It’s being hailed as the “Godzilla” of weight‑loss drugs and may rival (or even reduce the need for) bariatric surgery The Scottish Sun+4The Times+4The Sun+4.
Market Disruption & Competitive Edge
- If approved, retatrutide could quickly become a dominant force in the obesity and metabolic treatments market, helping Lilly gain substantial market share Heally+10Business Insider+10TipRanks+10.
- Its triple‑pathway mechanism and potentially better lean‑mass preservation may differentiate it from current GLP‑1 therapies Drugs.com+6Dr John Burns+6the biostation+6.
Broader Metabolic Benefits
- Beyond weight reduction, retatrutide may help with metabolic issues like fatty liver, blood pressure, and blood sugar control, enabling its use in broader metabolic and cardiovascular disease contexts Verywell Healththe biostation.
Safety & Side Effects
- Common adverse effects mirror other GLP‑1 agents: gastrointestinal symptoms (nausea, diarrhea, etc.) the biostationNew York PostThe Times.
- Some preliminary observations suggest less muscle loss and fewer visible side effects (like “Ozempic face”), making it more tolerable long-term—but robust, large-scale data is still needed New York Post.
- Media also notes skin-aging effects seen with weight-loss drugs; though not reported yet for retatrutide, caution remains The Scottish Sun.
Conclusion
Retatrutide is emerging as a groundbreaking contender in obesity pharmacotherapy:
| Feature | Summary |
|---|---|
| Mechanism | Triple agonist—GLP-1, GIP, and glucagon receptors |
| Efficacy | ~24% average weight loss at 48 weeks |
| Research Stage | Phase 3 trials ongoing (long-term, large cohort) |
| Estimated Launch | 2026 at earliest; 2027 more probable |
| Market Impact | Could redefine non-surgical obesity treatment and disrupt the GLP-1 market with superior efficacy and broader metabolic benefits |
As clinical trials continue and data accumulate, retatrutide may well usher in a new era in obesity and metabolic disease treatment—if all goes well.